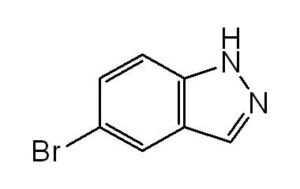
In the world of organic synthesis and pharmaceutical research, 5-Bromoindazole (CAS No: 53857-57-1) stands out as a valuable heterocyclic compound. Known for its unique structure and broad applicability in medicinal chemistry, this compound plays a key role in the development of advanced drug intermediates, materials, and chemical research.
This article explores the chemical properties, safe handling procedures, and research applications of 5-Bromoindazole.
Chemical Name: 5-Bromoindazole
CAS Number: 53857-57-1
Molecular Formula: C₇H₅BrN₂
Molecular Weight: 197.03 g/mol
Appearance: White to light beige crystalline powder
5-Bromoindazole belongs to the indazole family—aromatic nitrogen heterocycles known for their role in pharmacologically active molecules. The bromine substitution at the fifth position enhances its reactivity, making it a versatile building block for synthetic applications.
Property |
Value |
|---|---|
CAS No |
53857-57-1 |
Chemical Formula |
C₇H₅BrN₂ |
Molecular Weight |
197.03 g/mol |
Melting Point |
110–114°C |
Boiling Point |
Not determined (decomposes) |
Solubility |
Slightly soluble in organic solvents such as DMSO and ethanol |
Form |
Solid crystalline powder |
Purity |
Typically ≥ 98% (depending on supplier specification) |
These properties make 5-Bromoindazole suitable for precision reactions in laboratory and industrial settings.
The synthesis of 5-Bromoindazole often involves bromination of indazole derivatives under controlled conditions. The bromine atom serves as a reactive site for:
Cross-coupling reactions (e.g., Suzuki, Heck, or Sonogashira reactions)
Nucleophilic substitutions
Functional group modifications leading to more complex heterocyclic compounds
This reactivity makes it a preferred intermediate in drug discovery and material science.
Though not classified as a highly hazardous chemical, 5-Bromoindazole must be handled carefully under standard laboratory safety practices.
Personal Protective Equipment (PPE): Wear gloves, lab coat, and safety goggles.
Storage: Keep tightly closed in a cool, dry, well-ventilated area away from light and moisture.
Handling: Avoid inhalation, ingestion, or skin contact. Use only in fume hoods.
Spills: Clean up with appropriate absorbent material while wearing PPE.
Disposal: Dispose of in accordance with local chemical waste regulations.
GHS Classification: Not fully classified; handle as a laboratory reagent with care.
5-Bromoindazole is an important intermediate in the synthesis of biologically active molecules, including:
Anti-inflammatory agents
Anticancer compounds
Enzyme inhibitors
CNS-active drugs
Its indazole backbone is a key pharmacophore in many modern drug designs.
Chemists use 5-Bromoindazole as a building block to create structurally diverse indazole derivatives. The bromine atom allows selective substitution, leading to compounds with enhanced biological activity.
In advanced material applications, indazole derivatives contribute to the development of functional polymers, coordination complexes, and catalytic ligands with novel electronic or optical properties.
To maintain product integrity:
Store at room temperature (15–25°C)
Protect from moisture and direct sunlight
Keep container tightly sealed
Use desiccators or inert gas protection for long-term storage
Under proper conditions, 5-Bromoindazole remains stable for extended periods without significant degradation.
CAS No: 53857-57-1
HS Code: 29339990 (for heterocyclic compounds)
Transport Classification: Non-hazardous for air, sea, or ground shipping
Usage: For research and industrial purposes only. Not for human or animal consumption.
5-Bromoindazole (CAS No: 53857-57-1) is a highly versatile compound with wide applications in chemical synthesis, pharmaceuticals, and materials research. Its well-balanced chemical stability and reactive bromine site make it a valuable intermediate for innovative product development.
By following proper storage, handling, and safety guidelines, researchers can fully leverage its potential in cutting-edge scientific applications.