2-(Trifluoromethyl)benzenethiol, identified by CAS No. 13333-97-6, is an important organosulfur compound widely used in chemical synthesis and industrial research. Known for its unique trifluoromethyl substitution and thiol functional group, this compound serves as a valuable intermediate in pharmaceutical, agrochemical, and specialty chemical development.
This article explores its chemical properties, structure, applications, handling guidelines, and research significance.
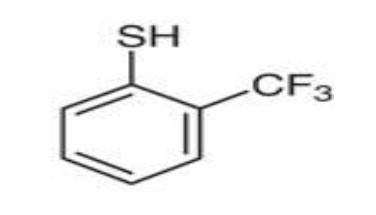
CAS Number: 13333-97-6
Chemical Name: 2-(Trifluoromethyl)benzenethiol
Molecular Category: Aromatic thiol
Functional Groups: Thiol (–SH), trifluoromethyl (–CF₃)
The presence of the electron-withdrawing trifluoromethyl group significantly influences the compound’s reactivity and stability.
2-(Trifluoromethyl)benzenethiol consists of a benzene ring substituted with a thiol group and a trifluoromethyl group in the ortho position. This structure offers:
Enhanced lipophilicity
Distinct electronic properties
High reactivity toward metal ions and electrophiles
Strong sulfur-based nucleophilicity
Compatibility with various organic solvents
Useful reactivity for thiol-based coupling reactions
This compound is frequently used as a synthetic intermediate in medicinal chemistry. Thiol-containing aromatic compounds are explored in:
Enzyme inhibition studies
Drug candidate modification
Structure–activity relationship (SAR) analysis
The trifluoromethyl group is valued for improving:
Biological activity
Metabolic stability
Environmental persistence of active compounds
As a result, CAS 13333-97-6 plays a role in agrochemical formulation research.
Used in the synthesis of sulfur-containing ligands
Applied in material science and functional chemical design
Acts as a precursor for advanced fluorinated compounds
Due to the reactive nature of thiols:
Store in a cool, dry, and well-ventilated area
Keep containers tightly sealed
Avoid prolonged exposure to air and light
Use appropriate PPE, including gloves and eye protection
Proper handling ensures stability, safety, and consistent research performance.
High-quality 2-(Trifluoromethyl)benzenethiol is typically supplied with:
Certificate of Analysis (COA)
Verified purity and batch traceability
Compliance with research and industrial standards
Sourcing from reliable suppliers is critical for accurate and reproducible results.
2-(Trifluoromethyl)benzenethiol (CAS 13333-97-6) is a versatile and valuable compound in modern chemical research. Its unique combination of a thiol group and trifluoromethyl substitution makes it an essential building block in pharmaceutical, agrochemical, and specialty chemical synthesis.
As demand for fluorinated and sulfur-based compounds grows, this chemical continues to support innovation across multiple research fields.