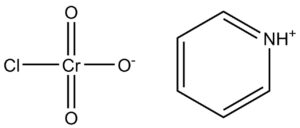
In the world of organic chemistry, oxidizing agents play a crucial role in driving transformations and enabling the synthesis of valuable compounds. Among them, Pyridinium Chlorochromate (PCC), identified by CAS No: 26299-14-9, has established itself as a versatile and widely used reagent. Known for its efficiency in mild oxidation reactions, PCC remains a staple in research laboratories and industrial applications.
Pyridinium Chlorochromate is an orange crystalline solid composed of pyridinium cations and chlorochromate anions. It was first introduced by Corey and Suggs in the 1970s as a safer and more selective oxidizing agent compared to earlier chromium(VI)-based reagents.
Chemical Formula: C₅H₆ClCrNO₃
Molecular Weight: 215.57 g/mol
CAS Number: 26299-14-9
Appearance: Orange crystalline powder
Acts as a mild and selective oxidant
Efficient for converting primary alcohols to aldehydes
Converts secondary alcohols to ketones without over-oxidation
Stable and easy to handle under laboratory conditions
Soluble in dichloromethane and other organic solvents
One of the most important uses of PCC is in the oxidation of alcohols. Unlike stronger oxidants, PCC allows selective conversion of primary alcohols into aldehydes without further oxidation to carboxylic acids.
PCC is widely applied in multi-step organic synthesis, where controlled oxidation is critical for building complex molecules such as pharmaceuticals, agrochemicals, and fine chemicals.
PCC plays a role in synthesizing intermediates for active pharmaceutical ingredients (APIs). Its ability to provide clean reactions with minimal byproducts makes it valuable in drug development.
In academic and industrial labs, PCC continues to be a reliable choice for method development, structure modifications, and fine-tuning of synthetic pathways.
While PCC is effective and convenient, it is still a chromium(VI) compound, which requires careful handling:
Use protective gloves, goggles, and lab coats.
Work in a well-ventilated fume hood.
Avoid contact with skin and inhalation of dust.
Dispose of waste as per hazardous chemical disposal guidelines.
With increasing environmental awareness, chemists also consider greener alternatives to PCC, such as:
Dess–Martin Periodinane (DMP)
Swern Oxidation
TEMPO-based oxidations
However, PCC continues to be preferred in many applications due to its cost-effectiveness and reliability.
Pyridinium Chlorochromate (CAS No: 26299-14-9) remains a cornerstone in organic synthesis, particularly valued for its selectivity, efficiency, and stability as an oxidizing agent. Whether in academic research or pharmaceutical development, PCC has proven its relevance for decades and continues to be a trusted reagent in modern chemistry.