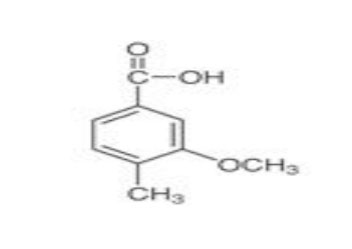
3-Methoxy-p-toluic acid (CAS No. 7151-68-0) is an aromatic carboxylic acid widely used as an intermediate in organic synthesis. Its functional groups—methoxy (–OCH₃), methyl (–CH₃), and carboxylic acid (–COOH)—make it a versatile compound in pharmaceutical, agrochemical, and specialty chemical industries.
Chemical Name: 3-Methoxy-p-toluic acid
CAS Number: 7151-68-0
Molecular Formula: C₉H₁₀O₃
Molecular Weight: ~166.17 g/mol
Chemical Class: Aromatic carboxylic acids
The compound features a substituted benzene ring with a methoxy group at the 3-position, a methyl group at the para position, and a carboxylic acid functional group. This structure contributes to both chemical stability and controlled reactivity.
Appearance: White to off-white crystalline solid
Solubility: Slightly soluble in water; soluble in organic solvents such as ethanol, methanol, and acetone
Acidity: Exhibits typical carboxylic acid behavior
Stability: Stable under recommended storage conditions
These properties allow for safe handling and effective performance in synthetic processes.
1. Pharmaceutical Intermediates
3-Methoxy-p-toluic acid is used as a key building block in the synthesis of active pharmaceutical ingredients (APIs), particularly in compounds related to anti-inflammatory and analgesic drugs.
2. Agrochemical Synthesis
The compound serves as an intermediate in the development of herbicides and plant growth regulators, contributing to modern agricultural solutions.
3. Specialty and Fine Chemicals
It is widely utilized in the manufacture of specialty chemicals, fragrances, and advanced intermediates requiring aromatic carboxylic acid functionality.
4. Research and Development
Chemical research laboratories employ this compound in studies involving substituted benzoic acids and structure-activity relationship (SAR) analysis.
The presence of both electron-donating (methoxy, methyl) and electron-withdrawing (carboxyl) groups allows 3-Methoxy-p-toluic acid to participate in esterification, amidation, and coupling reactions. This balanced reactivity makes it valuable for designing complex organic molecules in medicinal and industrial chemistry.
Use appropriate personal protective equipment (PPE)
Handle in a well-ventilated environment
Avoid direct contact with skin and eyes
Store in a cool, dry place away from incompatible materials
Standard chemical safety practices should always be followed.
7151-68-0 (3-Methoxy-p-toluic acid) is a versatile aromatic carboxylic acid with broad applications across pharmaceuticals, agrochemicals, and specialty chemical manufacturing. Its stable structure, functional versatility, and industrial relevance make it an essential compound for chemical professionals and researchers.