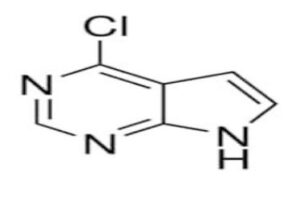
CAS 3680-69-1, scientifically known as 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine, is a heterocyclic compound widely recognized as a key pharmaceutical intermediate. With its unique pyrrolopyrimidine core, it plays an essential role in the synthesis of biologically active molecules, particularly kinase inhibitors used in cancer and autoimmune treatments.
As its applications grow in pharmaceutical R&D and manufacturing, understanding safe handling, storage, and best practices for its use becomes vital for both researchers and industrial chemists.
Property |
Details |
|---|---|
CAS Number |
3680-69-1 |
Chemical Name |
4-Chloro-7H-pyrrolo[2,3-d]pyrimidine |
Molecular Formula |
C₆H₄ClN₃ |
Molecular Weight |
153.57 g/mol |
Appearance |
Off-white to light yellow crystalline powder |
Purity |
≥98 % (common for pharmaceutical intermediates) |
Hazard Classification |
Irritant; harmful if swallowed or inhaled |
This compound acts as a building block for more complex molecules in medicinal chemistry, offering a stable structure for creating active pharmaceutical ingredients (APIs).
The compound is a core scaffold in the synthesis of Janus Kinase (JAK) and Tyrosine Kinase inhibitors—therapeutic classes critical for diseases such as:
Rheumatoid arthritis
Psoriasis
Myelofibrosis
Certain cancers
Pharmaceuticals like Ruxolitinib, Baricitinib, and Tofacitinib use intermediates derived from 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine.
Its reactive chloro substituent allows efficient nucleophilic substitution reactions, making it suitable for coupling with amines or heterocycles during multi-step synthesis.
This versatility makes it valuable in:
Antiviral drug development
Anti-inflammatory compounds
Oncology APIs
Academic and industrial laboratories frequently use CAS 3680-69-1 as a research reagent in drug discovery and structure–activity relationship (SAR) studies. It serves as a versatile fragment for creating new heterocyclic analogs.
According to supplier data (e.g., TCI Chemicals, ChemicalBook):
⚠️ Hazard Statements:
H302: Harmful if swallowed
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
Precautionary Statements:
P261: Avoid breathing dust or vapors
P280: Wear protective gloves, clothing, and eye protection
P305 + P351 + P338: Rinse eyes cautiously with water for several minutes if contact occurs
Handle only in well-ventilated areas or under a fume hood.
Avoid inhalation, ingestion, and direct contact with the skin or eyes.
Always wear:
Nitrile gloves
Lab coat or protective apron
Safety goggles
When weighing or transferring powder, use glove boxes or closed systems to prevent dust exposure.
Store in a tightly sealed container.
Keep in a cool, dry, and well-ventilated environment.
Protect from moisture, light, and heat.
Recommended storage temperature: 2–8 °C (refrigerated) for long-term stability.
Inhalation: Move the person to fresh air immediately; seek medical help if symptoms persist.
Skin contact: Wash thoroughly with soap and water. Remove contaminated clothing.
Eye contact: Rinse cautiously with water for several minutes. Seek medical attention.
Ingestion: Rinse mouth and obtain medical advice immediately. Do not induce vomiting.
Collect spills with absorbent materials (e.g., vermiculite).
Dispose of waste through licensed chemical waste handlers according to local regulations.
Never dispose of the compound down the drain or in regular trash.
Sector |
Usage Example |
|---|---|
Pharmaceutical Manufacturing |
Intermediate for kinase inhibitors |
Biotech Research |
Used in assay development and compound screening |
Chemical Synthesis Companies |
Base structure for custom molecule synthesis |
Academic Research Labs |
Structural modification for medicinal chemistry projects |
The compound’s ability to integrate into multiple synthesis pathways makes it indispensable across pharmaceutical R&D pipelines.
Although not classified as a major environmental hazard, responsible usage is encouraged:
Avoid uncontrolled release into water systems.
Adhere to REACH and OSHA chemical safety guidelines.
Maintain SDS documentation for each shipment and ensure compliance with local chemical regulations.
CAS 3680-69-1 (4-Chloro-7H-pyrrolo[2,3-d]pyrimidine) is a cornerstone compound in modern pharmaceutical synthesis. While it offers enormous value as a building block for advanced drug molecules, it must be handled with strict adherence to safety protocols to protect researchers and maintain regulatory compliance.
With growing demand for kinase inhibitor drugs and custom API synthesis, this compound’s relevance in global pharma is only expected to increase through 2025 and beyond.