Chemical compounds with halogenated heterocyclic structures play a crucial role in modern chemical and pharmaceutical research. One such important compound is 99839-30-2 (3-Bromo-4-chloropyridine 1-oxide). Known for its versatile reactivity and stability, this compound is widely used as an intermediate in advanced organic synthesis. This article explores its chemical properties, applications, and overall significance in research and industrial chemistry.
CAS Number: 99839-30-2
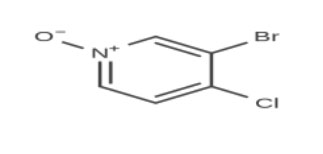
Chemical Name: 3-Bromo-4-chloropyridine 1-oxide
Molecular Formula: C₅H₃BrClNO
Compound Type: Halogenated pyridine N-oxide
The structure consists of a pyridine ring substituted with bromine and chlorine atoms, along with an N-oxide functional group. This combination enhances its reactivity, making it an excellent precursor for further chemical transformations.
Appearance: Typically a solid crystalline compound
Solubility: Soluble in common organic solvents
Stability: Stable under standard laboratory conditions
Reactivity: Highly reactive in substitution and coupling reactions
The presence of both halogen atoms and the N-oxide group allows for selective reactions, which is highly valued in synthetic chemistry.
99839-30-2 (3-Bromo-4-chloropyridine 1-oxide) is frequently used in the synthesis of active pharmaceutical ingredients (APIs). Its structure supports the creation of complex molecules used in drug discovery and medicinal chemistry.
The compound serves as a building block in the development of crop protection chemicals, including herbicides and fungicides, where heterocyclic compounds are essential.
It is widely used in laboratories for the synthesis of custom chemical compounds, supporting research in material science and industrial chemistry.
Due to the presence of bromine and chlorine substituents, this compound is ideal for Suzuki, Heck, and other cross-coupling reactions, enabling chemists to introduce diverse functional groups.
The importance of 99839-30-2 (3-Bromo-4-chloropyridine 1-oxide) lies in its versatility. The N-oxide group improves regioselectivity and reaction control, while the halogen atoms provide multiple pathways for modification. This makes it a valuable compound in:
Drug discovery programs
Advanced heterocyclic chemistry
Industrial-scale synthesis
Academic and R&D laboratories
Its reliability and adaptability contribute to faster development cycles in both pharmaceutical and chemical industries.
As with all halogenated chemical compounds:
Use appropriate personal protective equipment (PPE)
Handle in well-ventilated laboratory environments
Store according to recommended chemical safety guidelines
Always consult the Safety Data Sheet (SDS) before handling.
99839-30-2 (3-Bromo-4-chloropyridine 1-oxide) is a highly valuable compound in modern chemistry. Its unique structure, stable properties, and wide range of applications make it an essential intermediate for pharmaceutical, agrochemical, and specialty chemical synthesis. As research and industrial demands continue to grow, this compound remains a key contributor to chemical innovation.