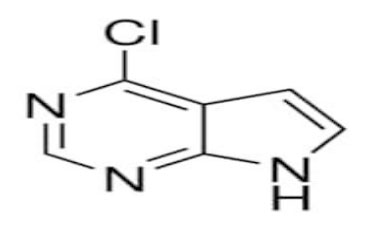
In the world of medicinal chemistry, heterocyclic compounds form the backbone of many groundbreaking drugs. One such valuable intermediate is 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine, identified by the CAS number 3680-69-1. This compound is widely used as a starting material for creating advanced pharmaceutical molecules, especially kinase inhibitors and antiviral agents.
This blog highlights its structure, properties, applications, and importance in chemical research.
4-Chloro-7H-pyrrolo[2,3-d]pyrimidine is a heterocyclic aromatic compound belonging to the pyrrolopyrimidine chemical family. Compounds in this class serve as vital intermediates for designing biologically active molecules due to their ability to interact with enzyme binding sites and biological receptors.
With a chlorine atom at the 4-position, this compound provides high reactivity, making it suitable for substitution reactions that yield diverse functional derivatives.
Parameter |
Details |
|---|---|
Chemical Name |
4-Chloro-7H-pyrrolo[2,3-d]pyrimidine |
CAS Number |
3680-69-1 |
Molecular Formula |
C₆H₄ClN₃ |
Molecular Weight |
153.57 g/mol |
Appearance |
Off-white to light beige crystalline powder |
Purity |
Typically 98% or higher |
Solubility |
Soluble in organic solvents like DMSO, methanol, and DMF |
High chemical reactivity due to the chloro substituent
Versatile intermediate for nucleophilic substitution
Excellent stability under normal storage conditions
Ideal scaffold for drug-design chemistry
4-Chloro-7H-pyrrolo[2,3-d]pyrimidine is widely used in the synthesis of:
Kinase inhibitors
Antiviral compounds
Anti-inflammatory agents
Cancer therapeutics
Its heterocyclic structure is ideal for forming small-molecule inhibitors that target specific biological pathways.
Its chlorine-substituted ring acts as an excellent electrophile, making it suitable for:
Substitution reactions
Coupling reactions
Formation of more complex heterocycles
This compound is a preferred choice for R&D teams exploring new therapeutic candidates. Its molecular framework allows easy modification to tune biological activity.
It contributes to the creation of molecules with:
Enhanced potency
Improved pharmacokinetics
Better selectivity for target enzymes
To maintain stability:
Store in a cool, dry place
Keep in tightly sealed containers
Avoid exposure to moisture
Handle using gloves, protective eyewear, and lab coat
Reliable intermediate for medicinal chemistry
High reactivity for synthetic versatility
Stable and easy to store
Widely available at high purity
Its combination of reactivity and structural importance makes it a staple in pharmaceutical discovery programs.
3680-69-1 (4-Chloro-7H-pyrrolo[2,3-d]pyrimidine) is an essential building block in the synthesis of cutting-edge therapeutic compounds. Its unique heterocyclic structure, reactivity, and broad application potential make it an indispensable reagent for researchers working on drug design, molecular development, and advanced organic synthesis.