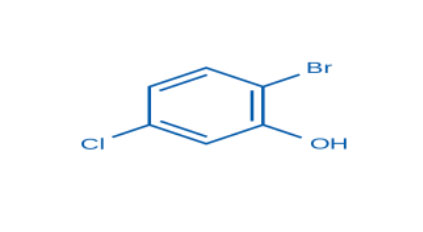
2-Bromo-5-chlorophenol (CAS No. 13659-23-9) is a halogenated phenolic compound widely used as an intermediate in organic and industrial chemistry. The presence of both bromine and chlorine substituents on the phenol ring enhances its reactivity and makes it valuable in pharmaceuticals, agrochemicals, and specialty chemical manufacturing.
Chemical Name: 2-Bromo-5-chlorophenol
CAS Number: 13659-23-9
Molecular Formula: C₆H₄BrClO
Molecular Weight: ~207.45 g/mol
Chemical Class: Halogenated phenols
The compound consists of a phenolic ring substituted with a bromine atom at the 2-position and a chlorine atom at the 5-position. The hydroxyl (–OH) group contributes to hydrogen bonding and acidic behavior, making the compound useful in further functionalization reactions.
Appearance: Light yellow to off-white solid
Solubility: Slightly soluble in water; soluble in organic solvents such as ethanol, ether, and acetone
Melting Point: Moderate, suitable for solid-state handling
Reactivity: Phenolic –OH group allows esterification and etherification; halogens enable coupling and substitution reactions
These properties make 2-Bromo-5-chlorophenol suitable for laboratory research and industrial-scale synthesis.
1. Pharmaceutical Intermediates
2-Bromo-5-chlorophenol is commonly used as a building block in the synthesis of pharmaceutical compounds, particularly in the development of antimicrobial and anti-inflammatory agents.
2. Agrochemical Manufacturing
The compound serves as an intermediate in the production of herbicides, fungicides, and pesticides, contributing to effective agricultural solutions.
3. Specialty and Fine Chemicals
It is used in the synthesis of advanced intermediates, custom chemicals, and performance materials for industrial applications.
4. Research and Development
Research laboratories utilize this compound for studying halogenated phenols and for developing novel organic molecules.
The dual halogen substitution pattern in 2-Bromo-5-chlorophenol provides excellent selectivity for cross-coupling reactions such as Suzuki, Heck, and Ullmann reactions. The phenolic functional group further expands its synthetic utility, allowing chemists to create complex molecular architectures efficiently.
Handle using appropriate personal protective equipment (PPE)
Use in a well-ventilated area or fume hood
Avoid skin contact and inhalation
Store in a cool, dry place away from strong oxidizing agents
Following standard safety protocols ensures safe handling and storage.
13659-23-9 (2-Bromo-5-chlorophenol) is a valuable halogenated phenolic intermediate with broad applications in pharmaceuticals, agrochemicals, and specialty chemical industries. Its versatile reactivity, structural features, and industrial relevance make it an important compound for chemical manufacturers and research professionals.